How to Effectively Name Ionic Compounds
Understanding Ionic Bonds and Their Components
To successfully navigate the realm of naming ionic compounds, it’s essential to grasp the foundational aspects of **ionic bonds**. An ionic bond forms through the transfer of **valence electrons** from one atom (often a metal) to another (typically a nonmetal), resulting in the formation of charged particles known as **cations** and **anions**. Cations are positively charged metal ions that lose electrons, while anions are negatively charged nonmetal ions that gain electrons. This interaction is crucial, as understanding the **charges of ions** helps determine the overall charge balance when writing **compound formulas** for binary and **polyatomic compounds**.
Defining Metal Ions and Nonmetal Ions
In naming ionic compounds, distinguishing between **metal ions** and **nonmetal ions** is vital. Metal ions usually exhibit fixed charges, particularly in Group 1 and Group 2 elements, which makes them easier to identify in nomenclature. Nonmetals, on the other hand, can take various oxidation states depending on the specific numbered group they belong to on the periodic table. When constructing names, the stability of these ions and their respective oxidation states can lead to errors, especially in mixed-valence systems such as transition metals.
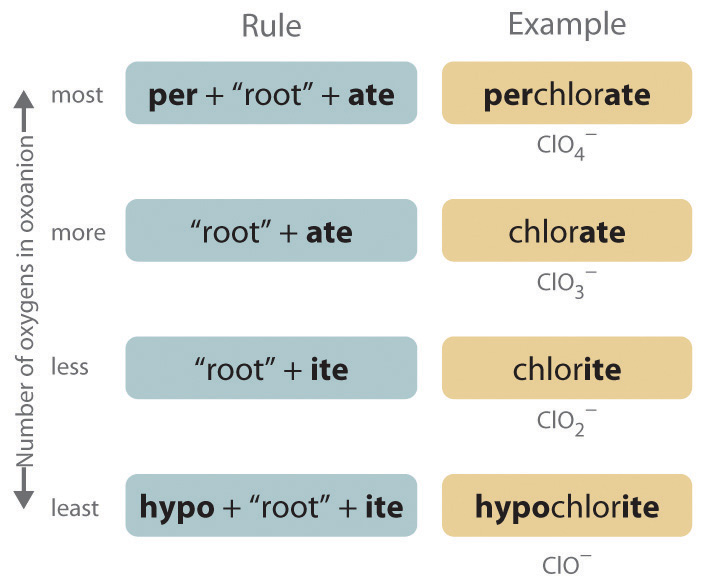
Binary Compounds vs. Polyatomic Ions
**Binary compounds** consist solely of two different elemental ions. For example, sodium chloride (NaCl) involves sodium (Na+) and chloride (Cl–). Naming these compounds follows straightforward rules. Conversely, **polyatomic ions**, like sulfate (SO42-) or nitrate (NO3–), comprise multiple atoms bonded covalently, yet they function as a single charged unit. Understanding how to identify and name these ions accurately with the appropriate nomenclature rules is crucial for students tackling chemistry projects.
Nomenclature Rules: Simple Steps to Remember
The methodical approach in naming involves set **nomenclature rules**, facilitated through various systems, including the classical and stock systems. Implementing these rules can ease the learning experience for aspiring chemists. The classical nomenclature draws from historical usage and often combines prefixes with suffixes to determine charges and composition, while the stock system emphasizes the use of oxidation states in naming metal ions, especially for transition metals that exhibit multiple charges.
Using the Stock System for Transition Metals
When dealing with transition metals, applying the **stock system** is crucial. Here, you indicate the charge of the metal ion using Roman numerals in parenthesis. For example, iron(II) oxide (FeO) implies two oxide ions (O2-) counterbalancing one Fe2+. Students must familiarize themselves with common transition metals and their typical oxidation states to grasp the principles of effective nomenclature.
Binary Compounds Naming Strategies
A straightforward strategy lies in the naming of binary compounds, where the **naming strategy** is relatively simple. The cation retains its elemental name while the anion’s name is altered by adding the suffix “-ide.” For instance, sodium and iodine form sodium iodide when combined as NaI. Understanding these basic conventions lays a robust foundation for more complex topics like **empirical formulas** and **compound ionization**.
Empirical and Molecular Formulas: Navigating the Differences
Recognizing the distinctions between **empirical formulas** and **molecular formulas** is crucial in chemistry education. An empirical formula represents the simplest whole-number ratio of elements, whereas a molecular formula indicates the actual number of atoms in a molecule. For example, the empirical formula for hydrogen peroxide is HO, while the molecular formula is H2O2. Understanding this discrepancy aids in precise **formula writing** and in conveying information effectively about **ionic compound properties**.
Examples of Common Ionic Compounds
Familiarity with **ionic compound examples** is imperative for mastering ionic nomenclature. Consider common substances: sodium chloride (NaCl), potassium sulfate (K2SO4), and calcium carbonate (CaCO3). These examples demonstrate how name structures reflect the composition and charges of the constituent ions and highlight essential naming conventions.
Identifying Common Polyatomic Ions
Utilizing **common polyatomic ions** is an effective tactic for students learning naming concepts. Memorizing key ions like hydroxide (OH–), sulfate (SO42-), and phosphate (PO43-) will soften the learning curve in understanding how to form ionic compounds and their corresponding names. Assigning these ions accurately supports students in avoiding **common misconceptions** that can arise during chemistry coursework.
Advanced Strategies and Common Mistakes
Mastering nomenclature can present challenges, leading to lingering **naming mistakes**. This necessitates the implementation of effective studying techniques, including collaborative learning through peer study groups, utilizing online resources, and engaging in hands-on chemistry labs to reinforce concepts. Practical examples reflect real-life applications of ionic compounds, demonstrating chemistry’s relevance.
Importance of Correctness in Nomenclature
The implications of **correctness in nomenclature** extend well beyond academic exercises. Mistakes in naming can lead to confusion or misunderstanding of ionic compounds, impacting various scientific industries from pharmaceuticals to materials science. Thus, students must hone their skills in order to communicate chemical information effectively, reinforcing both their educational endeavors and fostering strong future career pathways.
Common Misconceptions in Naming Ionic Compounds
One persistent issue among students learning ionic compounds is the reliance on misleading naming conventions. Often, students mix up rules of covalent and ionic nomenclature, resulting in inaccuracies. It’s essential to provide explicit examples that illustrate how covalent bonds differ from ionic bonds, through their structure, prefixes, and electron sharing. Addressing these misconceptions and providing clarity through additional educational resources will strengthen student comprehension and retention.
Key Takeaways
- Understanding the basic structure of **ionic compounds** is fundamental for correct naming.
- Applying the **stock system** works particularly well for identifying metals, especially transition metals with variable oxidation states.
- Familiarity with **common polyatomic ions** enhances students’ propensity to name compounds correctly.
- Correctness in nomenclature is vital for effective chemical communication and academic success.
- Engaging actively with educational resources, including named exercises and labs, can significantly aid understanding.
FAQ
1. What are the key components of ionic bonds?
The key components of **ionic bonds** involve the transfer of **valence electrons** from one atom to another, resulting in **cations** and **anions**. The attraction between these oppositely charged ions forms the fundamental structure of ionic compounds.
2. How do I determine charges for metal and nonmetal ions?
The **charges of ions** can generally be deduced from the periodic table. Metal ions usually lose electrons to become positively charged, while nonmetals tend to gain electrons to acquire a negative charge. Group elements often have a predictable charge; for example, alkali metals have a +1 charge.
3. What is the difference between empirical and molecular formulas?
The **empirical formula** represents the simplest ratio of elements in a compound, while the **molecular formula** gives the actual number of atoms present. For instance, glucose has an empirical formula of CH2O and a molecular formula of C6H12O6.
4. How do naming strategies differ for binary and polyatomic compounds?
Naming strategies for **binary compounds** involve using the metal’s name followed by the nonmetal’s name with an “-ide” suffix. In contrast, for **polyatomic compounds**, the name includes the polyatomic ion as it is listed without modification, such as ammonium sulfate (NH4)2SO4.
5. Why is correctness in nomenclature important for students?
Correct nomenclature allows for clear communication among scientists and significantly reduces the chances of misinterpretation in practical applications. Knowledge of proper naming conventions enhances a student’s credibility and professionalism in scientific fields.