“`html
Effective Ways to Calculate Percent Yield: A Simple Guide for 2025
Understanding Percent Yield in Chemistry
Percent yield is a crucial concept in **chemistry**, particularly when analyzing the efficiency of chemical reactions. It allows chemists to determine how much product was actually produced compared to the theoretical amount expected based on **stoichiometry**. To begin with, it’s important to grasp the **percent yield definition**: it measures the success of a reaction by comparing the actual yield (what you obtained from the experiment) to the theoretical yield (the maximum possible amount dictated by the balanced equation). For example, if a reaction theoretically yields 100 grams but only produces 80 grams, the percent yield is 80%. Understanding this concept lays the groundwork for effective **percent yield calculations** throughout experimental work.
The Percent Yield Formula
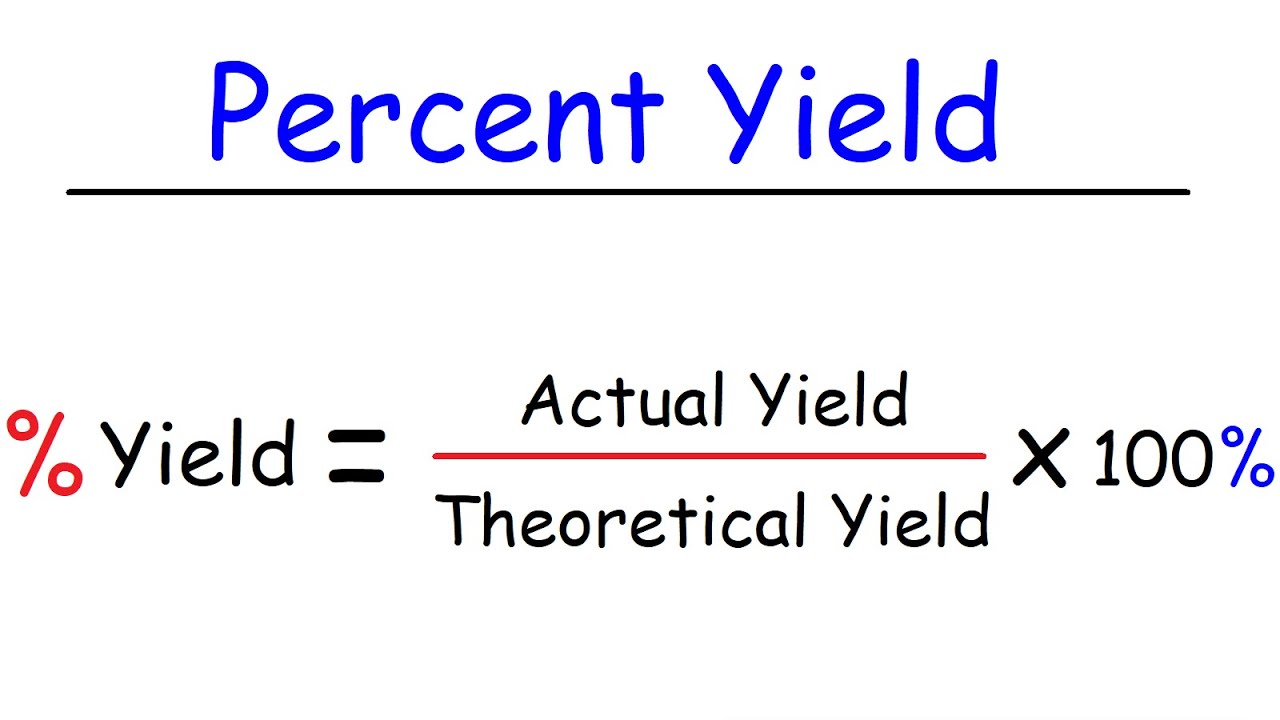
To calculate percent yield, two key measurements are required: the **theoretical percent yield** and the **experimental percent yield**. The **percent yield formula** is straightforward:
Percent Yield = (Actual Yield / Theoretical Yield) × 100
This simple formula plays a fundamental role in all sorts of chemistry experiments, and the calculations involved can reveal the underlying efficiency of given reactions. When working with the **percent yield in chemistry**, it’s vital to accurately measure the actual yield using precise methods to ensure reliable calculations and avoid discrepancies.
Factors Affecting Percent Yield
Several factors can influence percent yield, notably the purity of reactants, the extent of the chemical reaction, and any losses during product recovery. For example, if the **limiting reagent** is not accurately identified or if there are incomplete reactions, the actual yield may differ significantly from the theoretical yield, resulting in a lower percent yield. To optimize yield, one should focus on methods such as refining reaction conditions or improving reagent quality. Understanding how these factors interact can lead to higher **percent yield measurements**, ultimately enhancing the accuracy of experimental results.
Common Percent Yield Examples
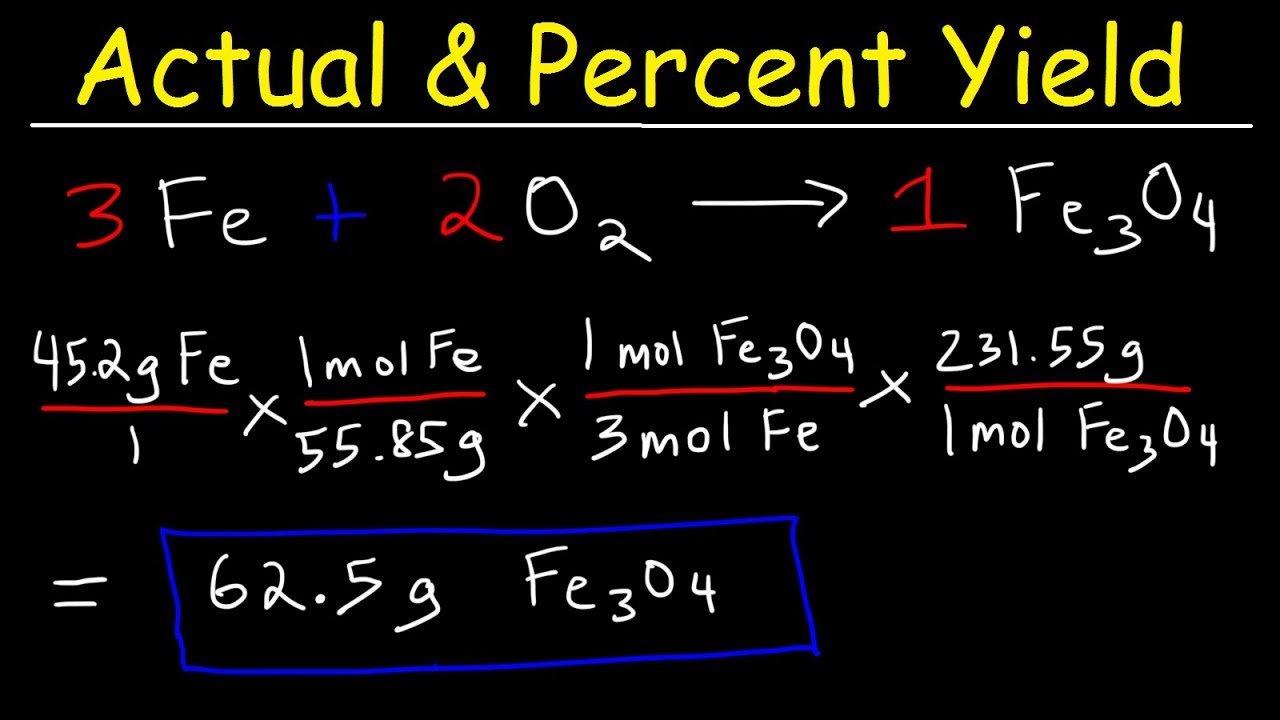
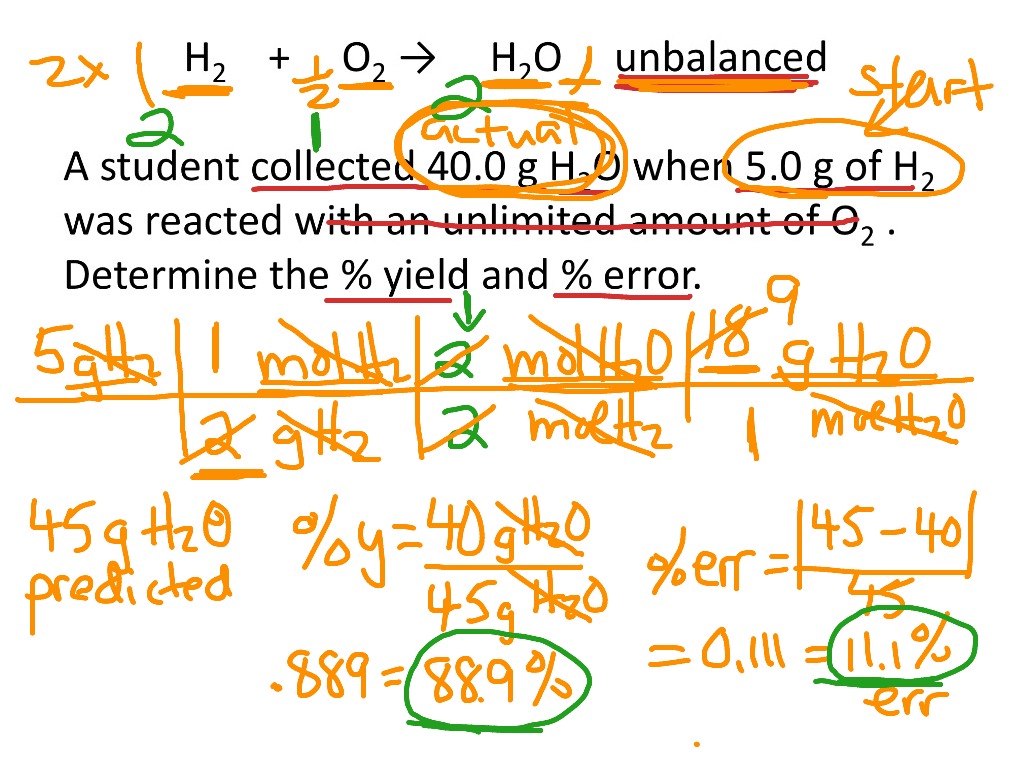
Let’s consider an example of a synthetic reaction between hydrogen and oxygen to produce water. The theoretical yield can be calculated from the balanced chemical equation, which predicts that 2 moles of hydrogen produce 2 moles of water under ideal conditions. If your actual yield turns out to be only 90 grams of water when 100 grams was expected, then applying the percent yield formula yields:
Percent Yield = (90 g / 100 g) × 100 = 90%
This process illustrates **percent yield examples** in practical terms, providing a clear reference point for scientists when assessing their reactions. From this, one can determine if the yield is satisfactory or whether adjustments are needed to improve product formation in future endeavors.
Calculating Theoretical and Actual Yields
Before diving into the practical aspects, it’s critical to differentiate between **theoretical vs. actual yield**. The theoretical yield is calculated based on **stoichiometry**, predicting maximum outcomes without considering loss factors. On the other hand, actual yield represents the quantity collected after an experiment, often varying due to various practical challenges encountered during the process. Understanding both yields can assist in identifying **percent yield relation to reactions** and further refining the synthesis strategies utilized.
How to Calculate Theoretical Yield
Calculating **theoretical yield** involves using molar ratios from a balanced equation. For instance, if a reaction requires 1 mole of zinc (Zn) to react fully with 2 moles of hydrochloric acid (HCl) to yield 1 mole of zinc chloride (ZnCl2), you can predict the theoretical yield of zinc chloride based on how much zinc you initially started with. Through these calculations, chemists can streamline their processes by setting higher expectations for their experimental planning.
Assessing Actual Yield
Actual yield determination is often fraught with challenges due to potential losses: dropout reactions, incomplete reactions, or even errors in measurement can drastically impact your final results. Accurate **yield assessments** often require well-established techniques in laboratory settings, including filtration, evaporation, and thorough cleaning of potential contaminants. These steps are essential to provide the most reliable data for calculating **experimental percent yield**, thus contributing to rigor in laboratory results.
Improving Percent Yield
To achieve better yields in your experiments, consider improving reaction conditions – this includes optimizing temperature and pressure, increasing the concentrations of reactants, or refining the pathways to minimize byproducts. Additionally, implementing techniques that reduce losses like better extraction methods or enhancing separation processes can significantly elevate your experimental performance. Emphasizing **improving percent yield** not only benefits individual projects but also contributes positively to advancements in chemical research and industry applications.
Significance of Percent Yield in Chemical Research
The importance of calculating percent yield extends beyond academic curiosity; it has profound practical implications. Understanding percent yield informs on economic viability in industries where yield efficiency is directly related to profit margins. Moreover, by revealing information about the **yield implications for reactions**, researchers can enhance their understanding of reaction pathways and molecular mechanisms, benefitting the overarching goals of innovation and efficiency.
Yield Implications for Reactions
In many compound synthesis projects, high percent yields often indicate efficient use of reactants, whereas low yields might trigger investigations into potential factors hindering reaction progress. Among these factors, reaction conditions (temperature, catalysts), the presence of impurities, and the reaction’s equilibrium state can all be scrutinized. Furthermore, frequent assessments of **yield in lab reports** enrich the narrative of scientific discovery with quantitative data and vital observations necessary for the ongoing refinement of experimental procedures, ultimately leading to improved understanding and application.
Linking Percent Yield to Industry Applications
Percent yield is particularly valuable in pharmaceutical and chemical manufacturing industries, where it’s critical to maximize output while minimizing costs. Companies consistently seek to optimize their **yield calculations** to not only improve production efficiency but also to ensure that they abide by safety and environmental regulations. By focusing on practical applications, stakeholders can assess production viability against changing market demands, thus aligning business strategies in a data-informed manner.
Advanced Techniques for Percent Yield Calculation
Advanced methods for calculating percent yield, such as using statistical process control (SPC) to monitor variations over time, guide syntheses and can aid in creating more robust processes. Such advanced techniques aggregate yield performance data to reveal trends that may otherwise be obscured by individual calculations. By investigating and standardizing these methods, institutions can enhance overall yield efficiency and accuracy in all research and production settings.
Key Takeaways
- Percent yield is critical for assessing the success of chemical reactions.
- Theoretical yield calculations must be understood in conjunction with actual yield to determine efficiency.
- Improving percent yield involves optimizing reaction conditions and procedures.
- Industries value percent yield for its connections to profitability and efficiency.
- Advanced techniques can streamline yield assessments for better results.
FAQ
1. What is the difference between theoretical yield and actual yield?
The theoretical yield is an estimate of the maximum amount of product that can be formed in a reaction based on the balanced chemical equation, while actual yield is the quantity collected from the experiment. Understanding this difference is crucial for calculating percent yield effectively.
2. How does one improve percent yield in experiments?
Improving percent yield can be done by optimizing reaction conditions, ensuring purity of reactants, and minimizing losses during product recovery. Regularly reviewing experimental procedures can lead to consistent increases in yield efficiency.
3. Why is percent yield significant in chemical reactions?
Percent yield provides insight into the efficiency of a reaction, guiding chemists in refining techniques, ensuring optimal use of resources, and improving overall waste management, which is crucial in industrial applications.
4. What are common sources of error that can affect percent yield?
Common sources of error include measurement inaccuracies, incomplete reactions, product loss during transfers, and reactions occurring at non-ideal conditions. Addressing these can support better percent yield outcomes.
5. How can percent yield impact industry profitability?
A high percent yield in production processes leads to a reduction in raw material costs and waste, directly affecting profitability. Industries that continuously assess and optimize their percent yield can increase their economic efficiency.
“`