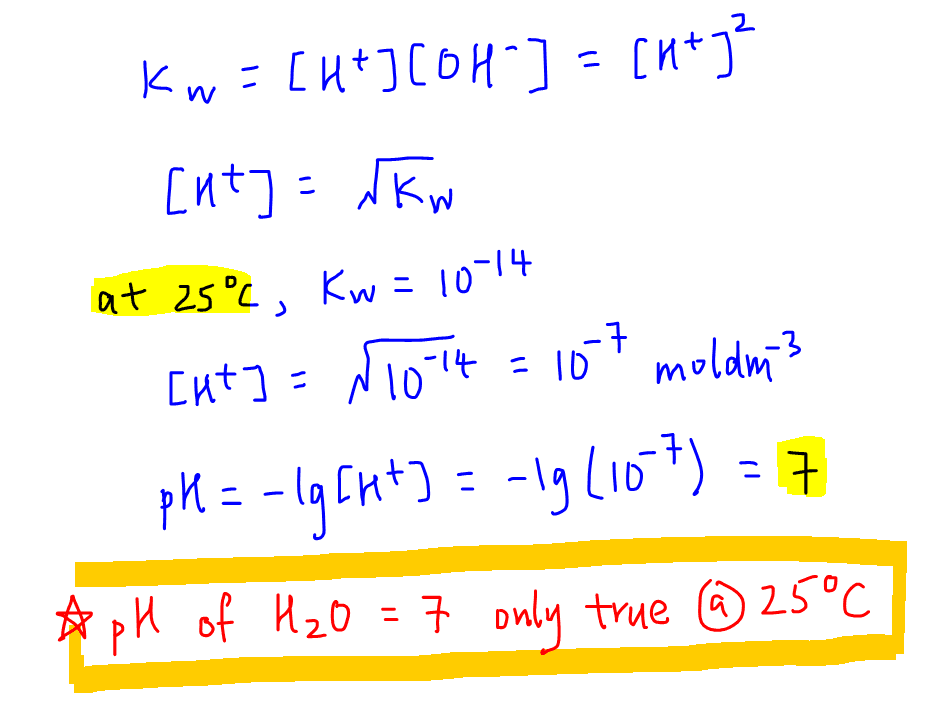Simple Ways to Calculate pH
Understanding pH: Definition and Importance
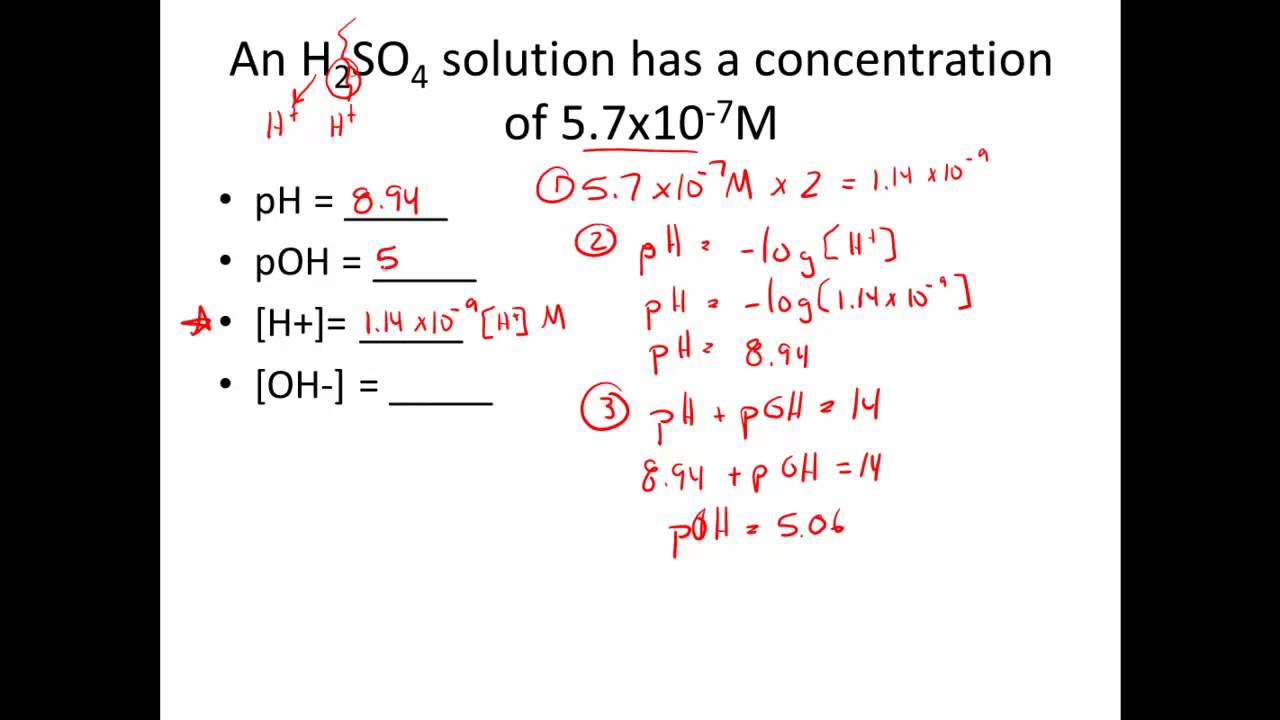
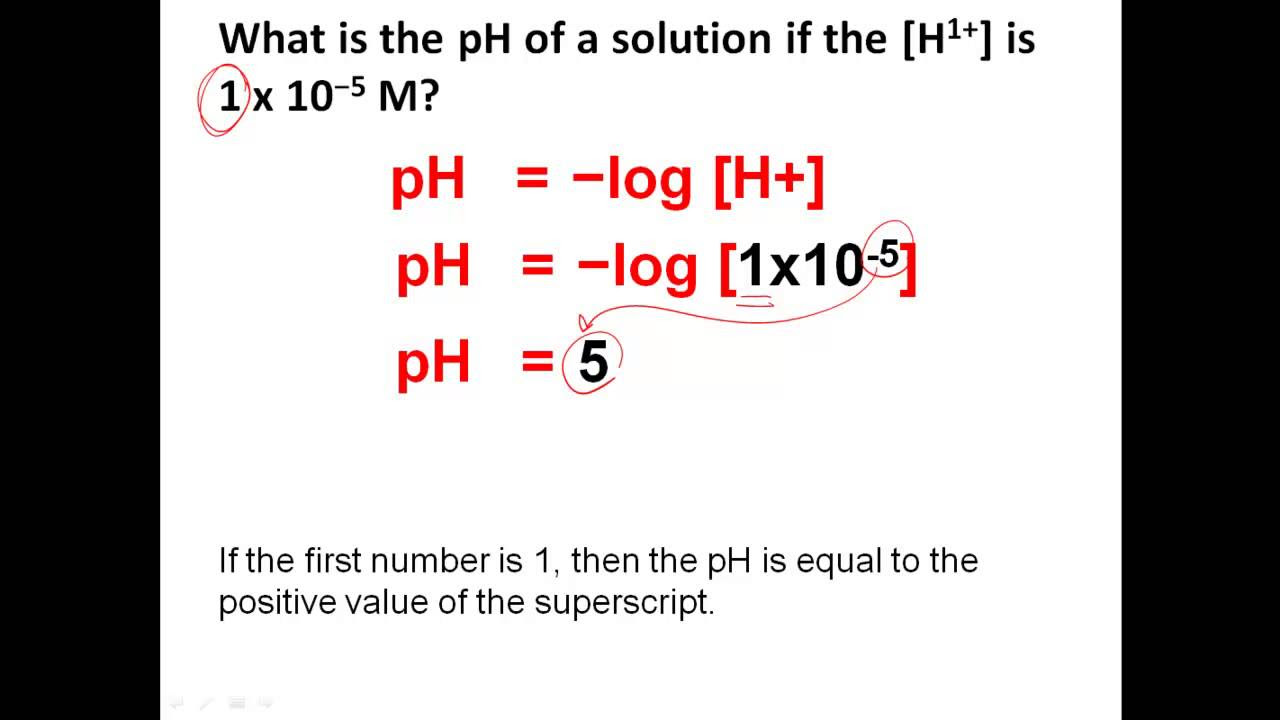
To grasp *how to calculate pH*, we must first define pH in chemistry. pH is a measure of the hydrogen ion concentration in a solution, representing its acidity or alkalinity. The *pH scale* ranges from 0 to 14, where values below 7 indicate acidity, values above 7 denote alkalinity, and a pH of 7 signifies neutrality. Understanding the pH of a solution is crucial for various fields like biology, agriculture, and environmental sciences—essentially wherever chemical reactions occur. This measure affects plant growth, human health, and even water quality, underlining the *importance of pH* in nature.
The pH Scale Explained
The *pH scale* is not linear; rather, it is logarithmic, which means that a one-unit change in pH represents a tenfold change in hydrogen ion concentration. For instance, a solution with a pH of 5 is ten times more acidic than one with a pH of 6. This characteristic makes pH not just a simple number but a *pH level* that conveys vital information about chemical interactions. In practical terms, knowing the exact pH can help in the formulation of products in industries ranging from pharmaceuticals to agriculture.
Role of pH in Health
The *importance of pH* extends into health and wellbeing as well. For instance, our bodies maintain a tightly regulated pH level within the blood (around 7.4). Deviations can affect enzyme activity, cellular function, and overall metabolism. When producing food or beverages, like beer or yogurt, understanding *pH levels* is critical, as it can influence both safety and flavor. Improper pH can lead to undesirable fermentation outcomes and affect the nutritional value of products.
Measuring pH: Techniques and Tools
When it comes to *measuring pH*, several methods and tools can be employed, each suited for different applications. These include traditional pH indicators, *pH meters*, and electronic pH meters, which provide varying degrees of precision and usability. In laboratories and industrial settings, *pH measurement techniques* often involve calibrated instruments for accurate readings, while simpler methods, like using *pH testing strips*, can be applied for quick assessments.
pH Indicators and Their Uses
*pH indicators* are substances that change color in response to pH changes. Commonly used indicators include litmus paper and phenolphthalein. When conducting experiments, it’s vital to select the right indicator, as not all indicators respond to the same pH range effectively. This approach is straightforward and often effective, especially for educational purposes or quick checks of liquids.
Using pH Meters for Accurate Measurements
*pH meters* are electronic devices designed to measure the voltage difference between two electrodes. This method allows for precise pH determination, making them commonly used in scientific research. Proper calibration of *pH meters* is crucial to ensure accuracy. Additionally, regular maintenance of these devices is essential for reliable readings and should be part of routine protocols when monitoring *pH levels* in any context, including agriculture and environmental analysis.
Practical Applications of pH Calculations
Calculating pH is not just an academic exercise; it’s incredibly relevant across various practical applications, whether in agriculture, environmental monitoring, or metabolic health. Understanding *acidity measurement* enhances one’s ability to control conditions for optimal growth in plants and crops, ultimately affecting food production and ecosystem balance. Furthermore, by evaluating *pH in water* systems, scientists can assess pollution levels and devise compatible solutions.
Exploring Soil pH Management
In agriculture, managing *soil pH* can determine the nutrient availability to plants. Each plant species has a preferred pH range, and deviations can hinder growth. For instance, many crops thrive optimally at a neutral to slightly acidic pH. Farmers often conduct soil tests to *detect pH levels*, which helps in the application of fertilizers and amendments to balance *acidic or alkaline substances*. This proactive approach ensures healthy crop yields and soil sustainability.
Real-World Example: pH in Food and Beverage Production
The concept of *pH in foods* plays a major role in food safety and fermentation processes. When creating products like cheese or fermented vegetables, producers closely monitor *pH levels* to ensure the safety and quality of the final product. For instance, during the fermentation of sauerkraut, controlling the pH is crucial for discouraging pathogenic bacteria while promoting desirable fermentative bacteria. This is yet another illustration of the *importance of pH* in everyday life.
pH Adjustment: Techniques to Optimize Conditions
Understanding how to adjust pH levels is critical across multiple sectors. For instance, in swimming pools, maintaining a balanced *pH level* is essential for safety and comfort. If the pH is too high or too low, it can lead to ineffective chlorine levels, causing skin irritation and discolored water. Techniques such as adding acids or bases to alter water pH are common practices. Additionally, *buffer solutions* can stabilize pH levels, enhancing the reliability of chemical reactions.
Common Methods for pH Adjustment
For adjusting pH levels, there are straightforward methods that anyone can apply. For acidic environments, adding *weak bases* like sodium bicarbonate helps raise pH, whereas adding acidic substances can lower it. Implementing these changes is crucial not only in pool care but also in agricultural practices. A well-calibrated approach leads to optimal growing conditions, benefiting both farmers and consumers.
The Benefits of Regular pH Testing
The *benefits of pH testing* cannot be overstated. Regular *pH monitoring* across agricultural land, swimming pools, or even individual gardens allows people to make informed decisions about necessary adjustments. Testing can be done using various methods ranging from electronic meters to simple litmus tests, making it accessible to farmers, home gardeners, and pool maintainers. Staying informed about *acidity vs alkalinity* through routine tests not only preserves health standards but also maximizes productivity.
Conclusion and Key Takeaways
Understanding and *calculating pH* equips individuals with essential knowledge applicable across numerous fields, including environmental science, agriculture, and health. From measuring solutions to interpreting pH results and making necessary adjustments, effective pH management plays a critical role in promoting quality and wellbeing. Remaining aware of *pH levels* is fundamental for anyone engaged in chemistry, agriculture, food production, or environmental stewardship.
FAQ
1. What is the significance of measuring pH in agriculture?
Measuring pH in agriculture is crucial as it influences nutrient availability for plants. Each plant species thrives within a particular pH range, thus understanding soil and water pH levels helps farmers apply the correct amendments to achieve optimal growth and yield.
2. How does the pH scale relate to acidity and alkalinity?
The pH scale ranges from 0 to 14, where values below 7 indicate acidity, values above 7 denote alkalinity, and a neutral pH of 7 represents balance. This scale is essential in determining the nature of substances in various environments.
3. What tools are advisable for home pH testing?
For homeowners, *pH testing strips* or digital pH meters are accessible and effective tools for assessing soil or water pH. These tools assist in managing plant health, aquatics, and even pool maintenance.
4. How often should pH levels be checked in pools?
Regular *pH monitoring* in pools, ideally at least once a week, is essential to maintain water quality and ensure safety for swimmers. Keeping the balanced pH helps stabilize chemical processes and minimize skin irritation.
5. Can pH affect food safety?
Yes, pH directly influences food safety, particularly in fermentation processes. A suitable pH level helps prevent harmful bacteria from thriving, while promoting beneficial bacteria that contribute to food preservation and safety.
6. What impacts can low pH have on organisms?
Low pH levels can cause various stressors in organisms, affecting physiological functions like enzyme activity and growth. In aquatic systems, for instance, low pH can lead to adverse effects on fish and aquatic plant life.
7. How is pH titration conducted in laboratory settings?
In laboratories, *titration to find pH* involves gradually adding a titrant to a solution until the desired pH is achieved, using indicators to visually confirm changes. This method provides precise pH values essential for scientific accuracy.
For further dilution of practical understanding, explore additional resources and techniques in pH assessment through the links provided: